Design and Engineering Angle: Advantages & Limitations of CGT Isolator Temperature Control Design Solution
News & Insights2024-01-10
Innovative Isolator equipment is being developed and used in cell and gene therapy (CGT) manufacturing, as more companies get involved in the emerging area of advanced therapy medicinal products (ATMPs). In the Cell & Gene Therapy (CGT) field, it is often heard that “the process is the product.” An optimal engineering design combined with identified CQA and CPP from the production process is crucial for ensuring the final product quality.

A CQA is “a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality” as stated in the ICH Q8 (R2) Guideline. The first step is to identify CQA in the cell and gene therapy production process, which generally includes appearance, cell quality/viability, potency, bioburden, purity and particulates, etc.
Based on the identified CQA, each production process step is also evaluated for its potential control parameters, and through a formal risk assessment, the criticality of each parameter is evaluated to determine the CPP that directly impacts CQA. Refer to ICH Q8, CPP is defined as “A process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired quality.”
The CPP for CGT production process generally includes temperature, PH and CO2 concentration, etc. For some process steps with open operation, such as filling, it is necessary to consider the operation in the isolator with a background of Grade C, and the influence of the temperature in the isolator on the product quality should be considered. If the temperature in the isolator is determined as the CPP after risk assessment, the temperature control strategy of the isolator should be considered during the design phase.
In this article, the advantages and limitations of five different CGT isolator temperature control design solutions from the perspectives of process system design and engineering design will be discussed.
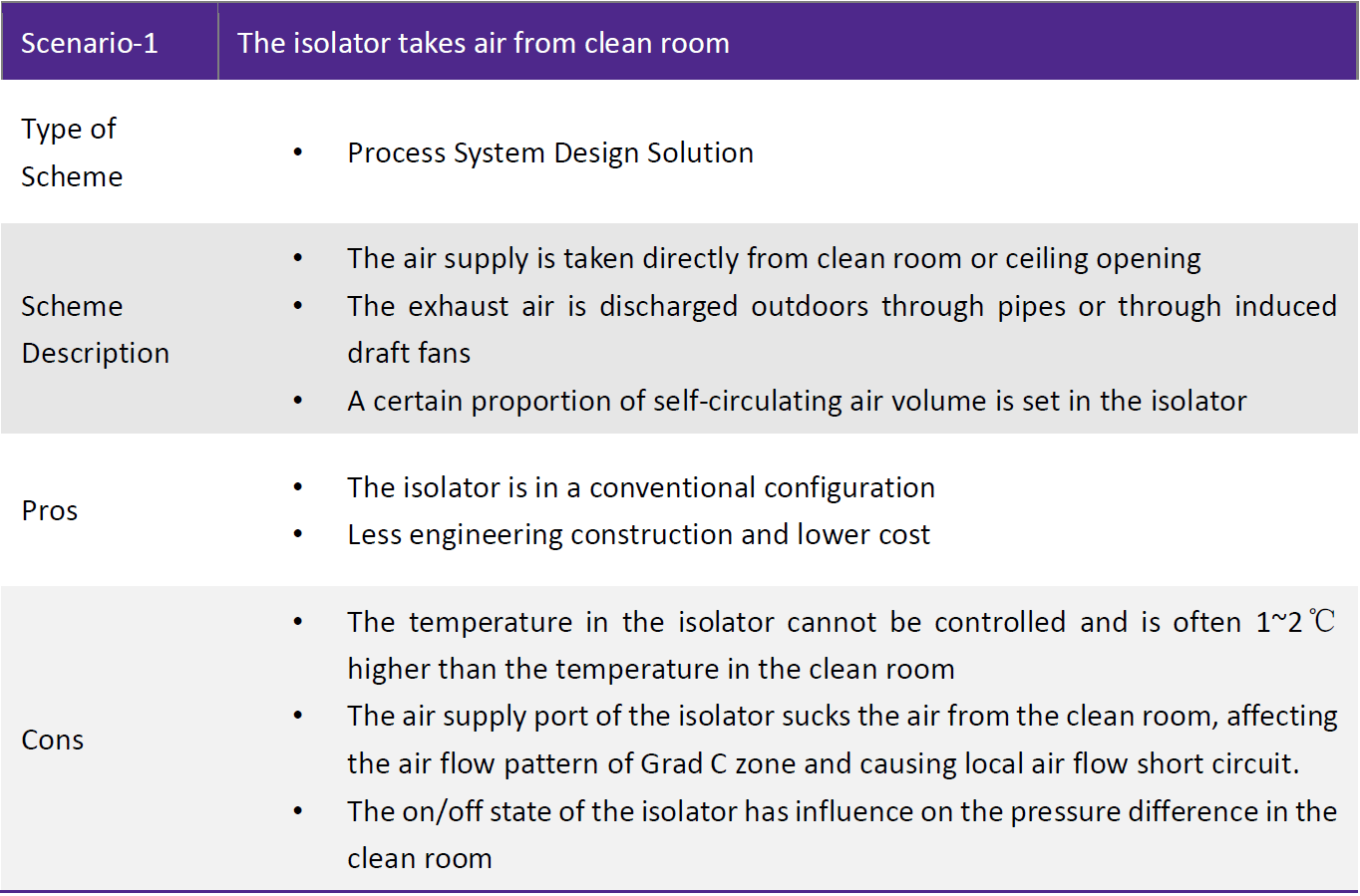
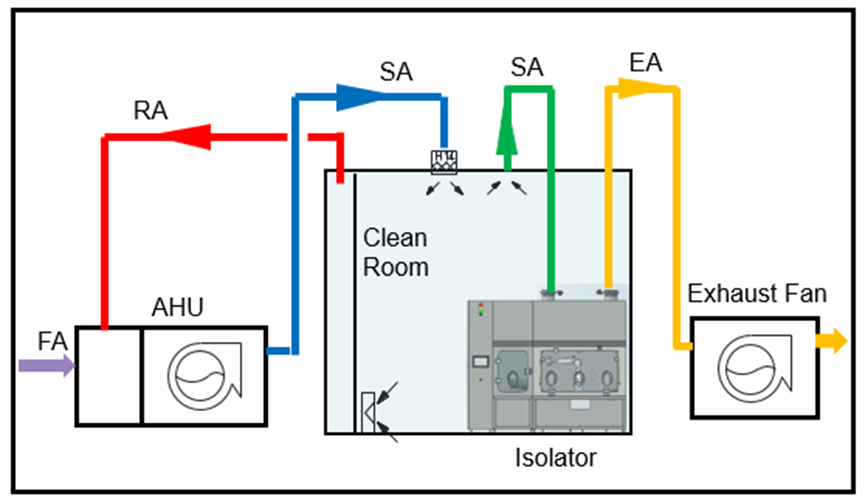
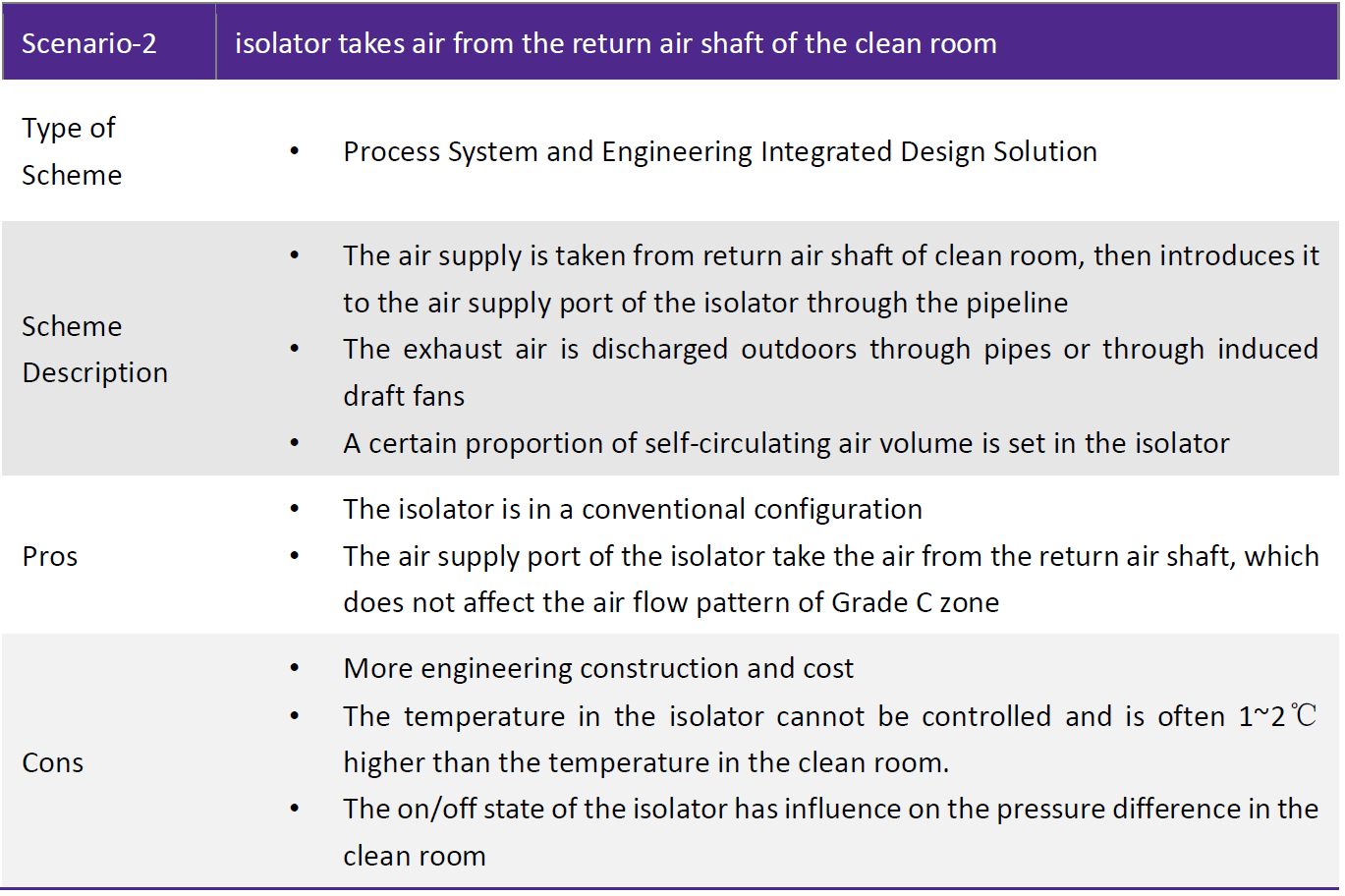
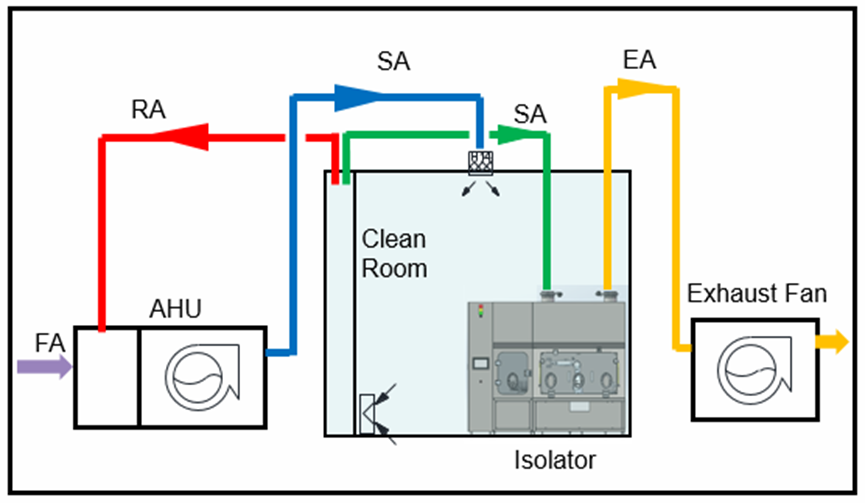
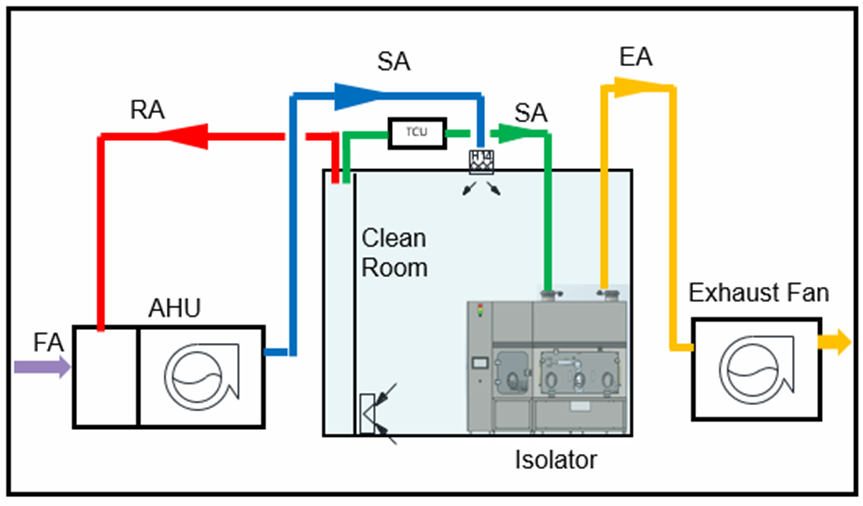
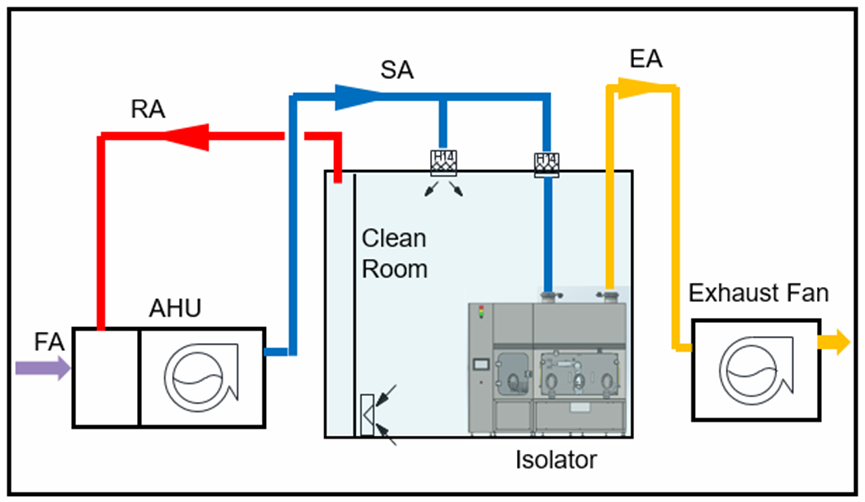
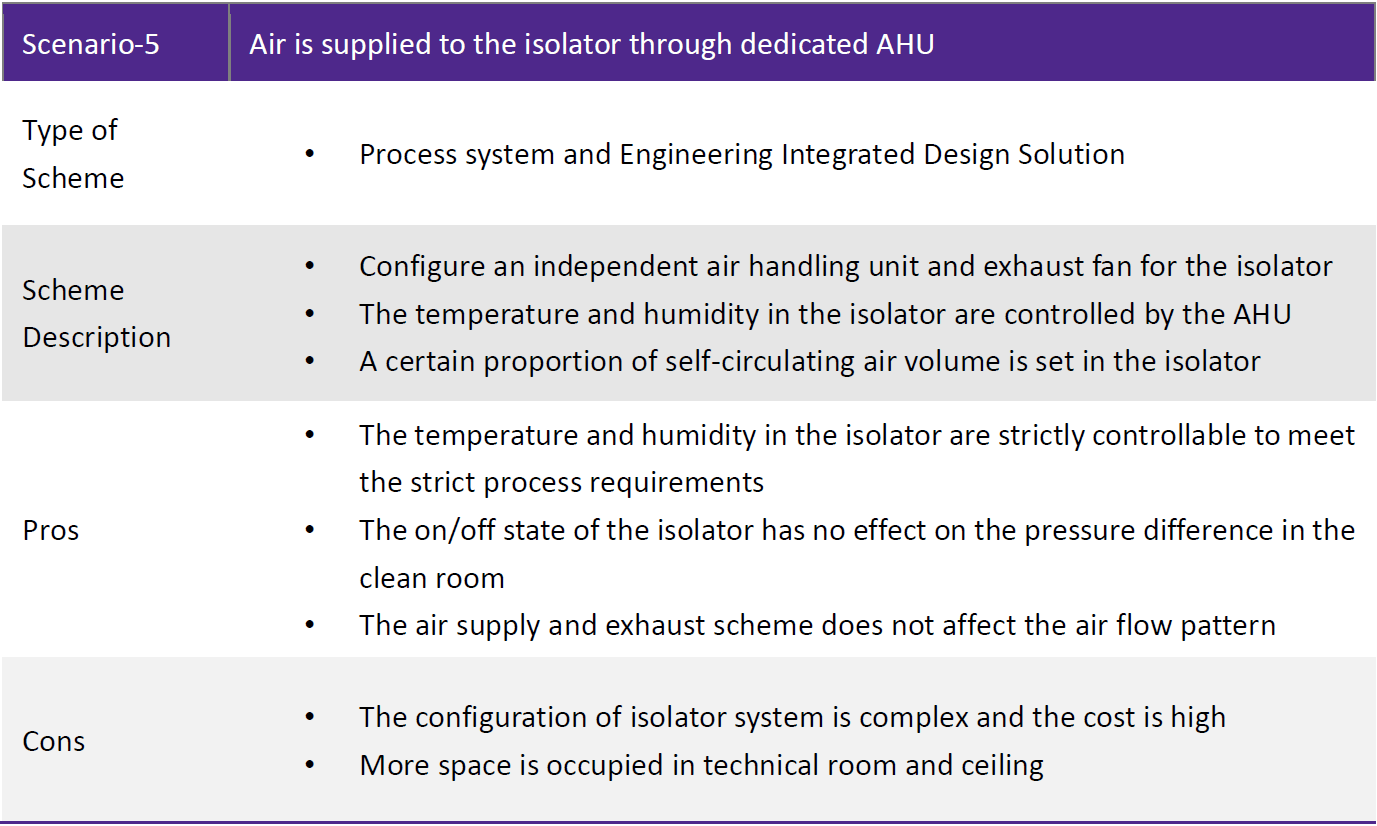
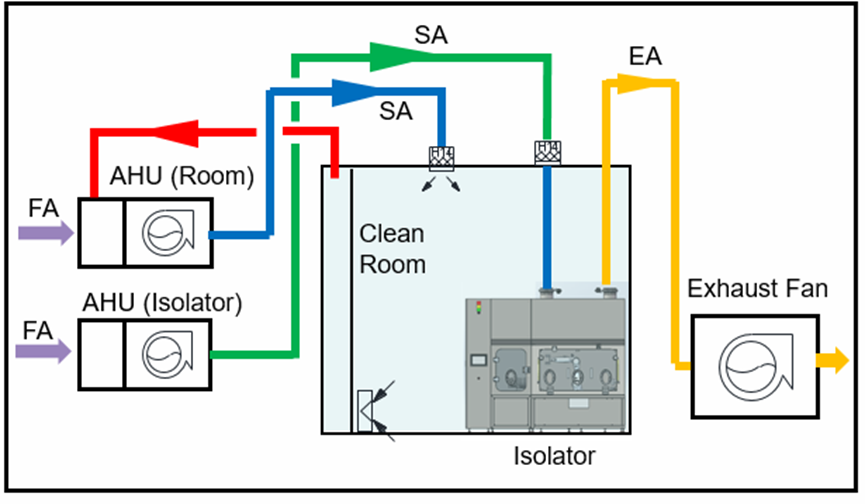
Conclusion
The isolator temperature control is closely related to the application conditions, CQA and CPP. It will be a cost-effective and comprehensive solution that integrates the design of the process system and engineering. Therefore, in the design stage, it is necessary to seek a professional consulting and design team to carry out the design combined with identified CQA and CPP from the production process to ensure the final product quality of CGT product.







 Search
Search 中文
中文
















